Anodizing
STANDARD ANODIZING
Aluminium always has a natural oxide layer on its surface. The anodizing process makes it possible to control the thickness of this oxide layer and therefore protect the metal against oxidization.
In addition, it is possible to use coloured pigments to colour the metal by using properties of the anodic layer. Anodisation Québec has a variety of colours. Simply enquire to find out which colours are available for your job.
METAL COLOURING
COMPOSITION OF THE OXIDE
Aluminium has a natural oxide layer. It forms by itself. The anodizing process makes it possible to control the thickness of this oxide layer and therefore protect the metal against oxidization.
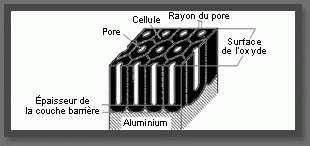
The oxide is made up of 2 layers: One acts as a barrier and one is porous. The barrier layer has a thickness of about 15 nanometres and the porous layer forms the rest. The thicknesses obtained through anodizing vary from 6 to 25 micrometres.
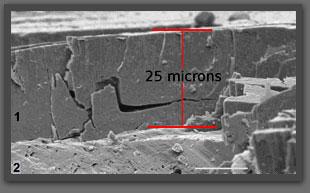
Oxide layer observed after a part has been broken. (1) oxide layer (2) aluminium (thickness of the layer: 25 micrometres)
In addition, it is possible to use coloured pigments to colour the metal by using properties of the anodic layer. Anodisation Québec has a variety of colours. Simply inquire to find out which colours are available for your job; colouring can be done in accordance with client specifications.
The oxide layer forms when the oxygen ions of the solution interact with the aluminium ions at the metal/electrolyte interface. The thicker the oxide layer becomes, the more difficult it will be to further increase it. The oxide is formed as hexagonal cells with a pore in the centre. During colouring, the colour pigments are found in these pores.
PENETRATION OF THE OXIDE
During anodizing, the oxide penetrates into the metal. The penetration of the oxide varies depending on the alloy, but there is normally a penetration of 35% and a swelling of 65%. It is important to know that this effect is inevitable during the treatment.
The oxide layer allows for a better abrasion resistance, because the aluminium oxide has a hardness of 200 to 350 Vickers (20 to 36 HRC).
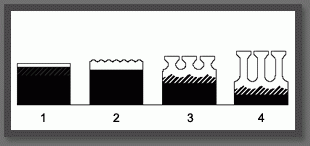
1 : initial thickness of the anodic layer
2 : beginning of the formation of pores
3 :penetration of the oxide layer and growth
4 : final state of the layer
CORROSION RESISTANCE
Anodizing treatment serves to protect against corrosion. The anodic coating that covers the part protects the metal against the oxidizing atmosphere. The oxide layer produced is in compliance with MIL-A-8625F standards.
COLOURING
The colouring process is an additional and optional procedure. It consists of the immersion of the anodized part in a solution containing a colourant. This is how the part takes on the desired colour. The only way to decolour the part is to destroy the oxide layer.
The photo to the right shows the various colours offered. They are not all available, but it is possible to order them.
SEALING
The process following colouring is called sealing. This process closes the pores by hydrating the formed oxide layer. Sealing allows for better corrosion resistance: Since the pores are sealed, there are a lot less sites susceptible to initiation of corrosion.
ELECTRIC PROPERTIES
It is possible to use anodized parts in high temperatures, but since the coefficient of thermal expansion of the oxide layer is around 5 times smaller than that of aluminium, it is recommended to proceed with successive increases in temperature. The same recommendations should be followed when cooling the layer. Here are some thermal properties of the anodic layer:
|
ELECTRIC PROPERTIES |
|---|
|
Resistivity For a non-hydrated layer of 50 micrometres |
| 4 x 10E15 ohms cm2/m à 20 °C |
| 8 x 10 E15 ohms cm2/m à 100 °C |
|
Breakdown voltage 20 to 40 volts/micrometre |
PROPERTIES AT HIGH TEMPERATURES
It is possible to use anodized parts in high temperatures, but since the coefficient of thermal expansion of the oxide layer is around 5 times smaller than that of aluminium, it is recommended to proceed with successive increases in temperature. The same recommendations should be followed when cooling the layer. Here are some thermal properties of the anodic layer:
|
PROPERTIES AT HIGH TEMPERATURES |
|---|
|
Coefficient of linear thermal expansion 5x10E-6 instead of 23x10E–6 of aluminium |
|
Thermal conductibility 21 to 63 W/m°C between 0 and 60 °C instead of 220 W/m°C for Al |
POROSITY OF THE LAYER
Since the oxide layer is porous, it can therefore be used as a layer allowing for better coating adherence on the part. Adhesive or paint can be applied. The molecules of these products insert themselves into the pores of the layer, allowing for better adherence.
HOW ALLOYS RESPOND TO SULPHURIC ACID ANODIZING
1000 series alloys (pure aluminium)
These respond well to the procedure. The more the metal is pure then the more the layers are transparent. To obtain an interesting appearance, the presence of iron and silicon must be avoided, even in small amounts.
2000 series alloys (aluminium-copper)
The copper present in this series will dissolve during anodizing and subsequently cause an aesthetically displeasing colour. Corrosion resistance will be weaker. The copper found in the second phase particles of the metal and during its dissolution prevents the coating of the oxide precipitate. Also, the abrasion resistance will be poor due to the non-uniformity of the oxide layer.
3000 series alloys (aluminium-manganese)
These alloys can be anodized well, but beyond 1%, the thick layers are less appropriate for aesthetics. The presence of manganese causes an undesirable grey-brown colour. Thus, the colouring procedure is combined with the treatment to improve aesthetics.
5000 series alloys (aluminium-magnesium)
These alloys can be anodized very well. The layers are relatively clear up to a 3% magnesium content. For higher contents, the layer loses a greyish colour, sometimes yellowish if the metal contains low amounts of manganese or chrome. These changes of colour are associated with the refractive index of the layer, which is affected by the presence of certain elements in the metal.
6000 series alloys (aluminium-magnesium-silicon)
These alloys can be anodized well and it is possible to obtain good results during colouration. Adapted homogenization treatments make it possible to obtain a very regular metallurgical structure, especially in relation to the size and the distribution of second phase precipitates. These treatments directly influence the final appearance of the metal after satin finishing (mat look) and anodizing. During large thickness anodizing (40 micrometres), the oxide layer loses a dark grey colour.
7000 series alloys (aluminium-zinc)
Alloys that contain a high amount of copper (example: the 7075 alloy) pose some problems during anodizing, because they react the same way as 2000 series alloys. However, the grades that contain a small amount of copper are well suited for sulphuric acid anodizing (example: the 7020 alloy).
OTHER FACTORS THAT INFLUENCE THE ANODIZING QUALITY
In the case where an aluminium part is cast from a sand mould or had underwent a sand surface treatment, the aesthetics of the colouring procedure will not be at their best. An exceedingly high presence of silicon must be avoided in order to obtain a quality anodizing and uniform colour.
Other impurities can influence the quality of the oxide layer. Before anodizing, it must be certain that there is no longer any oil on the aluminium parts. If this is not the case, the layer will not form. Also, the presence of oil will contaminate the basin and will affect the other parts.
ANODIZING DIFFERENT ALLOYS
Each type of alloy responds differently to anodizing. During anodizing, sets of the same alloy should be treated together in order to have good control over the thickness. The alloys must always be well identified to avoid confusion in the basins.
EFFECT OF HELICOILS OR INSERTS
The presence of helicoils or inserts different than the base material in an alloy will contaminate the baths. Subsequent treatments will be of poor quality. Therefore, these parts must be removed before anodizing.
FLAWS THAT APPEAR DURING ANODIZING
The anodizing treatment can make certain characteristics of alloys used stand out.
Microstructure
All crystalline metals have a microstructure which is unique to the treatment that they have underwent. During the treatment, the metal is put in contact with aggressive solutions that can make this microstructure stand out.
Porosities
The different surface treatments that parts can undergo can hide certain flaws of the metal. Surface irregularities that were polished mechanically can resurface following anodizing. After the first anodizing steps, these flaws reappear and affect the quality of the colouring procedure. So small white spots are present on the surface after the final procedure. The following photo shows the effect of re-apparition of flaws:
Points of contact
In order to anodize a part, it is essential to have points of contact on the part. These serve to transfer the electricity to the part so that the anodizing can take place. The oxide will not form on these parts and it will be possible to see white spots. However, the points of contact are chosen with the aim of preserving the aesthetics of the part.
Welds
When parts are welded, they have underwent a significant thermal process that changes the microstructure of the metal. This change will affect the colour of the anodic layer. This means that the differences in colour between aluminium and the welds will be apparent.
Photo 1 shows a welded part. This part is then anodized (photo 2). It is possible to see that the weld is darker than the part. Also, the area that underwent the thermal effect is a bit whiter. In photo 3, the part has been coloured. Once again, it is possible to notice that the colour is not uniform around the periphery and in the weld.
Colour
It is important to specify when one same colour is desired for a set of parts of the same alloy. From one treatment to another, there are always small variations in the procedure and this causes slight differences in colour.
Note: It is normal to have a slight variation in colour for large orders.
MAINTENANCE OF ANODIZED PARTS
Regular maintenance of anodized surfaces is essential for removing accumulations of dirt of deposits. Proper maintenance will also prevent the appearance of spots on the parts. It is important to not use just any cleaning technique, because if an inappropriate product is used, it can damage the anodized layer.
Here is a table that describes the proper maintenance techniques :
The use of coarse abrasive pads (examples: steel wool or sand paper), very strong cleaning products (dish-washing and laundry detergents) and basic or acidic products will ruin the aluminium layer. Aluminium oxide is stable for pH levels between 5 and 10. For pH levels exceeding this interval, the oxide will be attacked.
|
TYPE OF PROBLEM |
PRODUCT |
PROCEDURE |
|
Dust deposit |
Warm water and soap or detergent |
Thoroughly wipe the excess water with a clean cloth to prevent deposits of calcium salts. |
|
Grease deposit |
Organic solvent (ex. : kerosene) |
Use the solvent to rinse the surface with a clean cloth and dry to prevent streaks |
|
Large deposit of dirt or grime |
Light abrasive material and water |
Clean, rinse and dry the surface |
|
Deposit of tough stains |
Fine abrasive pads |
Gently rub, remove metallic fibres, rinse and dry |